Safety of Janus Kinase Inhibitors in Patients With Inflammatory Bowel Diseases or Other Immune-mediated Diseases: A Systematic Review and Meta-Analysis
Pablo A. Olivera,1,* Juan S. Lasa,1,2,* Stefanos Bonovas,3,4 Silvio Danese,3,4 and Laurent Peyrin-Biroulet5
1Gastroenterology Section, Department of Internal Medicine, Centro de Educación Médica e Investigaciones Clínicas (CEMIC), Buenos Aires, Argentina;
2Gastroenterology Department, Hospital Británico de Buenos Aires, Argentina;
3Department of Biomedical Sciences, Humanitas University, Milan, Italy;
4IBD Center, Department of Gastroenterology, Humanitas Clinical and Research Center, Milan, Italy; and
5INSERM NGERE and Department of Hepatogastroenterology, Nancy University Hospital, Lorraine University, Vandoeuvre-lés-Nancy, France
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e18. Learning
Objective: Upon completion of this CME activity, successful learners will be able to identify the safety profile of Janus Kinases (JAK) inhibitors in patients with Inflammatory Bowel Disease and other Immune-mediated Diseases.
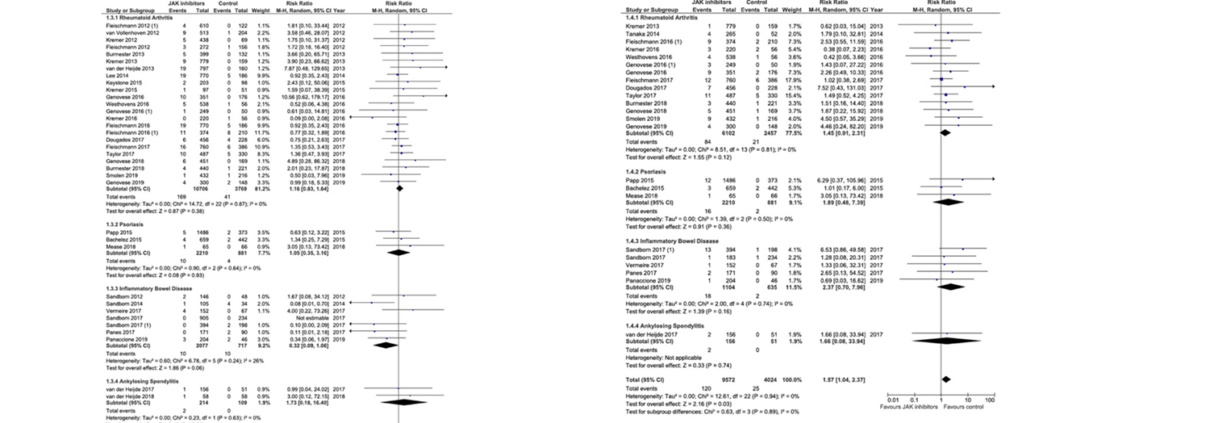
BACKGROUND & AIMS: Inhibitors of Janus kinases (JAKs) are being developed for treatment of inflammatory bowel diseases and other immune-mediated diseases. Tofacitinib is effective in treatment of ulcerative colitis, but there are safety concerns. We performed a systematic review and meta-analysis to investigate the safety profile of tofacitinib, upadacitinib, filgotinib, and baricitinib in patients with rheumatoid arthritis, inflammatory bowel diseases, psoria-sis, or ankylosing spondylitis. METHODS: We searched the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials from January 1, 1990, through July 1, 2019. We performed a manual review of conference data-bases from 2012 through 2018. The primary outcome was incidence rates of adverse events (AEs) and serious AEs. We also estimated incidence rates of serious infections, herpes zoster infection, non-melanoma skin cancer, other malignancies, major cardiovascular events, venous throm-boembolism, and mortality. We performed a meta-analysis, which included controlled studies, to assess the relative risk of these events. RESULTS: We identified 973 studies; of these, 82 were included in the final analysis, comprising 66,159 patients with immune-mediated diseases who were exposed to a JAK inhibitor. Two-thirds of the included studies were randomized controlled trials. The incidence rate of AEs was 42.65 per 100 person-years and of serious AEs was 9.88 per 100 person-years. Incidence rates of serious infections, herpes zoster infection, malignancy, and major cardiovascular events were 2.81 per 100 person-years, 2.67 per 100 person-years, 0.89 per 100 person-years, and 0.48 per 100 person-years, respectively. Mortality was not increased in patients treated with JAK inhibitors compared with patients given placebo or active comparator (relative risk 0.72; 95% confidence interval 0.40–1.28). The meta-analysis showed a significant increase in risk of herpes zoster infection among patients who received JAK inhibitors (relative risk 1.57; 95% confidence interval 1.04–2.37). CONCLUSIONS: In a systematic review and meta-analysis, we found an increased risk of herpes zoster infection among patients with immune-mediated diseases treated with JAK inhibitors. All other AEs were not increased among patients treated with JAK inhibitors.
Keywords: NMSC; IBD; Immunosuppression; Small Molecule.
Fuente:
https://www.gastrojournal.org/article/S0016-5085(20)30011-1/fulltext